- 19 Posts
- 25 Comments
Do it! it is easy to do at home! Just wear some gloves and safety glasses, those jars can easily shatter during the heating process if you use too hot of a heat source. I recommend a glass top electric stove, or put some kind of metal plate between your jar and the burner to help spread out the heat. Once you seal the jar, take it off the heat right away, so it doesn’t build pressure. I boiled mine for a few minutes before sealing to try and get some of the devolved gasses out, and lightly set the lid on top to help the steam push out all the air.
Yes definitely. The pressure will drop along the vapor pressure curve all the way to the triple point, gently boiling all the way if you remove the heat from the vapor and not directly from the water.
Looks great! Nice work! What mount do you use?
Anything for posterity
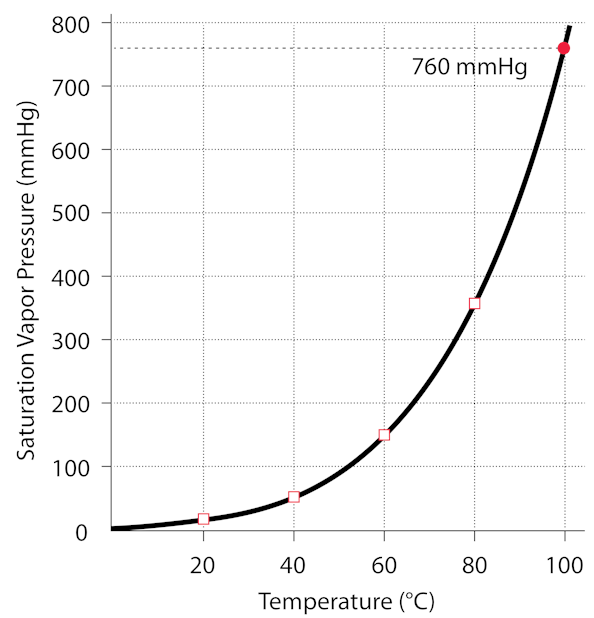
Exactly. it was bottled at atmospheric pressure while it was boiling, so 1 atm and 100 degrees C. Check this graph to see the relationship between the water’s temperature and it’s pressure in the jar (since there is no air, only water vapor). If the vapor is condensed, then the pressure drops below the curve on the graph, that is, the pressure in the jar is lowered below the vapor pressure of the water. Any time the pressure is below the vapor pressure, the water will boil, releasing vapor, until the pressure is equal to the vapor pressure. The pressure does not become negative, it is still positive, just lower than the vapor pressure at the given temperature. You can get below the vapor pressure curve by changing the temperature too, which is what we usually do when boiling water at a pressure near 1 atm (760mmHg)
http://hyperphysics.phy-astr.gsu.edu/hbase/Kinetic/watvap.html#c2
(1 atmosphere is ~760mmHg)
a slight aside, there is an important difference between the total pressure of the air, and the partial pressure of water vapor in the air. Inside the jar, the two are equal, but in a dry location (not humid) the partial pressure of water vapor is usually less than the vapor pressure of water at that temperature, but since the total large pressure of the atmosphere would not allow a pocket/bubble of very low pressure water vapor to form inside the bulk water, the water cannot boil, but it will evaporate at the surface anyway until the partial pressure of water is equal to the vapor pressure (very humid).
I decided to translate the worksheet into GLSL code on shadertoy. It was really cool to see the gradients and sub-coordinate systems represented by the intermediate variables in the calculation. Smoke and mirrors. Maybe you might have some insight into some of the calculations. https://www.shadertoy.com/view/cllBzM
Any ideas?
I was thinking of doing three separate GOL simulations, one on each RGB channel, and letting the colors mix that way into like 6 colors. right now, I clamp the pixel brightness values to 0 or 1, so that’s why it’s black/white, or rather black/green.

 1·10 months ago
1·10 months ago2nd on the keep notes suggestion. I work on lots of unrelated projects, and each time I end up learning a bunch of new command line utilities, so I try to leave behind a text file describing some of the most useful commands I’d discovered that day. Usually helps me come back to a project and not be back at square one every time.

 1·10 months ago
1·10 months agoI thought there was something slightly peculiar about the narration.

 111·11 months ago
111·11 months agoI hear you, but genetic change at the level of these diseases and traits can take on the order of hundreds of thousands of years or more to accumulate into meaningful trends. Social society is a part of that process, in the way it might be for other social animals. If social dynamics tend to result in communities harboring vulnerable individuals, then there is probably some selective advantage to that behavior, not the other way around.

 232·11 months ago
232·11 months agoThis is a common misconception. These traits are not likely due to modern medicine (which is very, very new compared to the scale of human evolution). The environment plays a big role, but there is always a distribution of traits in a normal population, some good, some bad. Not to mention that what we might be self-selecting for must change very rapidly as civilizations rise and fall, preferences shift like the winds, and ethics rapidly evolve. I think this misconception can be dangerous, because of what you mentioned. Eugenics.

 1·11 months ago
1·11 months agoThe internet is a series of tubes.

 2·11 months ago
2·11 months agoThanks, I fixed it

 4·11 months ago
4·11 months agoYou’re getting it.

 2·11 months ago
2·11 months agoThis exact thought has been tossing around in the back of my mind since I read it. I was trying to figure out the legitimate reasons for not wanting to live in a city, and as far as I can tell, there are a few. But I’m not sure if it’s worse than living 45min away from downtown in some suburb and always feeling stranded without gasoline, no-matter if you are at home(too far away from the grocery store), in the city, or somewhere on the long highway connecting the two. There is no place for a person without a car really, not for more than a couple hours.
I just read it, what a good article. I’m not sure I see the vision at the end, but I totally agree with the way he describes every aspect of the problem.
Some of my favorite excerpts, in addition to the one you quoted:
“The typical American devotes more than 1500 hours a year (which is 30 hours a week, or 4 hours a day, including Sundays) to his [or her] car. This includes the time spent behind the wheel, both in motion and stopped, the hours of work to pay for it and to pay for gas, tires, tolls, insurance, tickets, and taxes .Thus it takes this American 1500 hours to go 6000 miles (in the course of a year). Three and a half miles take him (or her) one hour. In countries that do not have a transportation industry, people travel at exactly this speed on foot, with the added advantage that they can go wherever they want and aren’t restricted to asphalt roads.”
You’ll observe that automobile capitalism has thought of everything. Just when the car is killing the car, it arranges for the alternatives to disappear, thus making the car compulsory. So first the capitalist state allowed the rail connections between the cities and the surrounding countryside to fall to pieces, and then it did away with them.
These splintered cities are strung out along empty streets lined with identical developments; and their urban landscape (a desert) says, “These streets are made for driving as quickly as possible from work to home and vice versa. You go through here, you don’t live here. At the end of the workday everyone ought to stay at home, and anyone found on the street after nightfall should be considered suspect of plotting evil.” In some American cities the act of strolling in the streets at night is grounds for suspicion of a crime.
No means of fast transportation and escape will ever compensate for the vexation of living in an uninhabitable city in which no one feels at home or the irritation of only going into the city to work or, on the other hand, to be alone and sleep.
I decided to give it a try over the weekend on a road trip, through the apps Organic Maps and Go Map!! I really liked Go Map!! except that it crashes occasionally, and won’t restart until your reinstall it :( loosing all the GPS tracks and unsubmitted data :(( If it was more stable, I’d recommend it to everyone.




















Crawling and indexing lemmy inter-instance would be an incredible boon to discoverability on the platform.